INDEX
(Not linked)
SCROLL DOWN FOR RHYMING STUDY AIDS
Acid Reactions, Generic
Acids, Strong and Weak
Air Liquefaction Fractions
Allotropes of Carbon
Amphipathic Detergents
Amphipathic Detergents
Amphipathics
Avogadro’s Law
Enantiomers
Exothermic
Halogen Names
Helium to Fluorine
Halogen Names
Helium to Fluorine
Isotopes
Le Chatelier’s Principle
Moles and Molar Solutions
Noble Gases
Periodic Table of the Elements
Permanganate Equations
Permanganate Equations
Phases of Matter
Phenolphthalein
Phenolphthalein
Saccharides
Solutions
Sucrose and Glucose
Sucrose and Glucose
Attributions
at the end of this page.
Acid
Reactions, Generic
Acid + base = salt + water
Acid + alcohol = ester + water
When acids attack our bases
We neutralize their assault
With a salt.
But when acids try alcohol
Si ester time!
By Alan Beech
Students may confuse ‘concentrated’ and ‘dilute’
solutions
of acids in water with the ‘strength’ (a property) of
the acid.
Strong acids you may see
In a chemist's laboratory.
Are nitric, hydrochloric,
Sulfuric and perchloric.
Two weak acids we pick
Acetic and citric.
Acetic and water we call vinegar
Citric and water like lemonade are.
Acids strong in aqueous solution
Have a fully ionic distribution.
Acids weak dissolve in water too
Ions they make are relatively few.
By Alan Beech
Although only a small percentage of air by
volume, these
noble gases are available as air is liquefied on a
large scale.
Out of liquid air
Fractions we prepare
Neon and argon,
Krypton and xenon.
Distilled fractions
Of liquefactions.
By Alan Beech
Allotropes
of Carbon
DIAMOND GRAPHITE
By Itub
Diverse forms of carbon are known
Called allotropes, they stand alone.
Carbon with covalent bonding find
In diamond, hardest mineral mined.
Crystals of graphite are readily shed
As pencil carbon, wrongly called lead.
Amorphous (powdered) carbon is gleaned
As soot when any chimney is cleaned.
BUCKYBALL
By Mstroeck
Found by modern science means
New forms of carbon, fullerenes.
Nanotube and buckyball allotrope
With important future uses, we hope.
The newest exciting fullerene
Is a hexagon lattice called graphene
One atom thick with bizarre properties
Strong and having good conductivities.
By Smokefoot
If it mixes in aqueous goop
With a lipid attracting group
And one wanting water to grope
We call it detergent or soap.
By Alan Beech
Amphipathic compounds have a fat-attracting (lipophilic) group at one end
and a water-attracting (hydrophilic) group at the other end. Water-miscible
amphipathic compounds are soaps or detergents.
Lipid loving groups are lipophilic
Water loving groups are hydrophilic
When both are on a single chemic-
Water loving groups are hydrophilic
When both are on a single chemic-
The chemical is amphipathic.
By Alan Beech
Avogadro said two gases
In equal volume glasses,
At the same Temp and P
Equal count of molecules see.
Using PV = nRT
An ideal gas mole at STP
Occupies by Avogadro’s Law
Liters twenty two point four.
By Alan Beech
By Chirality_with_hands
Dashes are added in the poem to aid pronunciation.
Mirror imaged compounds chiral
Bend rays of plane polarized light
Toward a left or right hand spiral
Levo-(left) or dextro-(right)
Each is an optical isomer
Or else an en-ant-i-om-er.
By Alan Beech
Exothermic
In an exothermic reaction
Heat is a product of the action.
By Alan Beech
Halogen
Names
Don't
mispronounce the name of iodine.
First four
halogens rhyme with bean
Fluorine, chlorine, bromine, iodine.
Fluorine, chlorine, bromine, iodine.
By Alan Beech
The symbols of elements 2 to 9
of the Periodic Table can be
sung to the first line of the song, Frère Jacques. After hydrogen
the next eight elements are helium, lithium, beryllium, boron,
carbon, nitrogen, oxygen, fluorine. Their symbols are He Li Be
B C N O F.
sung to the first line of the song, Frère Jacques. After hydrogen
the next eight elements are helium, lithium, beryllium, boron,
carbon, nitrogen, oxygen, fluorine. Their symbols are He Li Be
B C N O F.
Chemical elements 2 to 9 sound off
Make them sound like Helly Beb-k-noff
‘Frère Jacques’ notes in treble clef
f and g and a and f.
By Alan Beech
Isotopes
By BruceBlaus
The number of protons in an element is its atomic
number. The number of
neutrons may vary. Each variant is an isotope. The
relative atomic
mass of an element is an average of the masses of its isotopes.
An element found in any amount
It’s atoms all have the same proton count.
But different atoms of one element
With differing numbers of neutrons present.
An element with several neutrons cope.
We call each one of them an isotope.
We call each one of them an isotope.
By Alan Beech
Le Chatelier’s Principle has application
To temp, press, vol and concentration.
When an equilibrium reaction rearranges
The system tries to combat the changes.
By Alan Beech
The molecular weight of a compound in grams (formula
weight) is a mole.
It contains 6.022 x 1023 molecules, the
Avogadro number. One mole
dissolved in pure water and made up to a liter is a
molar solution.
For every chemical you find
With a formula well defined
Weigh in grams the formula weight
A mole of chemical you create.
In a mole many molecules see
Six times ten to twenty three*
A number named for Avogadro
Next to a molar solution we go.
Dissolve a mole in water pure
At the ambient temperature
Place in a liter volumetric flask
Top to the mark to end the task.
*(Note added here for fussy few:
It’s really six point oh two two).
It’s really six point oh two two).
By Alan Beech
By Pumbaa
The naturally occurring noble gases are helium (He),
neon (Ne), argon (Ar),
krypton (Kr) [Pron. Krip-ton],xenon (Xe) [pron. zeenon]
and radioactive
radon (Rn) [Pron. ray-don]. In the atoms of the noble
gases, the outer "valence"
shell is filled full of electrons, so
they seldom take part in chemical reactions.
For the same reason they are
mon-atomic, i.e. each atom is a molecule.
With shells of electrons fully pack'd
The noble gases seldom react.
Helium we waste in a toy balloon
We'll value more than gold dust soon.
Neon next to balloonacy
Makes the red strip lights you see.
Argon next to neon share,
Is almost one percent of air
But you'd never know it's there.
Krypton next in the noble plan
A gas, not the planet of Superman.
Xenon heaviest noble able
To have a nucleus that's stable.
Radon, radioactive gas
Sometimes found in homes, alas.
By Alan Beech
Periodic Table of the Elements
Mendeleev,
a scientist, was able
To
uncover the Periodic Table.
After
comparing all of their properties
He
sequenced known elements in families.
By
atomic weights (now we say masses)
He
left some gaps but put them in classes.
When
elements he predicted were found
They knew Mendeleev’s work was sound.
Moseley
found that atomic numbers defined
Nuclear
proton numbers in elements assigned.
The
Table by atomic number was rearranged
It’s
value increased and some places changed.
By Alan Beech
Potassium permanganate may appear in hard ‘equation balancing’ questions.
Others examiner favorites are
potassium dichromate K2Cr2O7, potassium
ferrocyanide K4Fe(CN)6 and potassium ferricyanide K3Fe(CN)6.
Two moles of Pot. Permanganate
(With molar mass one fifty eight)
Will oxygen times three donate.
Dissolved in water alkified
2KMnO4 divide
K2O, 2MnO2, 3O’s inside
Equation balancing will guide.
(With molar mass one fifty eight)
Will oxygen times three donate.
Dissolved in water alkified
2KMnO4 divide
K2O, 2MnO2, 3O’s inside
Equation balancing will guide.
By Alan Beech
Phases of Matter
By Yelod
When molecules jostle each other
Like troops in formation together
Or penguins in very cold weather
We call that phase a solid.
Like troops in formation together
Or penguins in very cold weather
We call that phase a solid.
When molecules add heat energy
Decreased hydrogen bonding we see
They flow into vessels easily
They flow into vessels easily
We call that phase a liquid.
To molecules still more heat supply
Until like birds way up in the sky,
They break their hydrogen bonds and fly.
We call that phase a gas.
Until like birds way up in the sky,
They break their hydrogen bonds and fly.
We call that phase a gas.
By Alan Beech
Phenolphthalein (pron. Fee-nol-thay-lean) is an
end-point
indicator used in titrating strong acids with strong bases.
Phenolphthalein indicator,
Magic color change creator.
Solutions are quite colorless
When at pH 8 or less.
Above, it turns magenta-red,
In acid, all the color's shed.
By Alan Beech
The saccharides (pron. Sack-a-rides) are sugars, a
class of water-soluble organic compounds.
Saccharide is sugar-speak
A word derived from Early Greek.
Every science student knows
Names of sugars end with "ose".
Glucose and dextrose are the same
As Shakespeare said "What's in a name"?
Glucose, fructose and ribose besides
Are three of the monosaccharides.
Plants make sugars to store energy
We harvest them to set sugars free.
By Alan Beech
Solutions
Solvent dissolves solute
Solute appears gone
Stir for uniformity
To get a solution.
With water as solvent
Dissolve solute salt
When salt no longer seen.
When salt no longer seen.
The solution is saline.
Solutions share the solvent’s phase
But they may change in other ways.
Color change one can easily see
Less obvious changed conductivity.
Saline freezes at temperature
Less than water, that’s for sure.
It’s boiling point is higher, these
Are called colligative properties.
The formula for fructose fix
It is C six H twelve O six.
For glucose the formula is the same
Its atoms shift so does its name.
The sugars in food we ingest
Change into glucose we digest,
Then the glucose circulates free
To bring cells lots of energy.
Sucrose taste is just as sweet
Derived from sugar cane or beet,
Its the same compound as sweet as heaven,
C twelve H twenty two O eleven.
By Alan Beech
Uranium
Atomic Bomb
By Fastfission
An atomic bomb
Gets its energy from
Fusion or fission
With neutron emission.
U two thirty eight, the kind
Of most uranium ore mined,
But isotope U two thirty five
By gas centrifuge we derive.
If a fast neutron hits
U two three five, it splits
And two new atoms see
Plus released energy.
This U atomic fission
Sheds neutrons in addition
When speeding from this action
They start a chain reaction.
By Alan
Beech
Valence
Stable
molecules are neutral electrically
Their
electrons float in orbits of energy,
So
the number of nuclear positive protons.
Equates
to orbiting negative electrons
Lighter
elements try to create
A
filled up valence shell of eight.
Filled
shells are inert chemically
And
that is what they try to be.
Halogen
atoms F, Br and Cl
Need
one electron to fill that shell
Alkali
metals Li, Na and K
Have
a spare they can give away.
Sodium
catches fire when wet
Chlorine,
war gas, bad as you get.
They
react together with a flash
Form
sodium chloride (salt) as ash.
Chlorine
wants to add on
That
sodium electron
As
side by side they sit
It
flashes to get it.
By Alan Beech
ATTRIBUTION OF IMAGES
Allotropes of carbon
By Diamond_and_graphite.jpg: User:Itub derivative
work: Materialscientist (Diamond_and_graphite.jpg File:Graphite-tn19a.jpg)
[GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0
(http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
Allotropes of carbon Buckyball.
By Mstroeck at en.wikipedia Later versions were
uploaded by Bryn C at en.wikipedia. [GFDL (www.gnu.org/copyleft/fdl.html) or
CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], from Wikimedia
Commons
Amphipathic Detergents.
"Soap&Detergents" by
Smokefoot - Own work. Licensed under Public domain via Wikimedia Commons
Amphipathic Phospholipids
"Phospholipid
Chemicalmakeup" by Veggiesaur - Own work. Licensed under Creative Commons
Attribution-Share Alike 3.0 via Wikimedia Commons
Enantiomers
"Chirality with hands" by
Chirality_with_hands.jpg: Unknownderivative work: -- πϵρήλιο ℗ - Chirality_with_hands.jpg. Licensed under Public
domain via Wikimedia Commons
Isotopes
"Blausen 0530
HydrogenIsotopes" by BruceBlaus - Own work. Licensed under Creative
Commons Attribution 3.0 via Wikimedia Commons
Noble Gases.
"Electron shell 010 Neon
- no label" by commons:User:Pumbaa (original work by commons:User:Greg
Robson) - http://commons.wikimedia.org/wiki/Category:Electron_shell_diagrams
(corresponding labeled version). Licensed under Creative Commons Attribution-Share
Alike 2.0-uk via Wikimedia Commons
Phases of Matter
By Yelod - Wikimedia Commons * Yelod - Wikipedia (En) * ילוד - ויקיפדיה העברית (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Uranium Atomic Bomb.
By Fastfission in the public domain.
By Yelod - Wikimedia Commons * Yelod - Wikipedia (En) * ילוד - ויקיפדיה העברית (Own work) [CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Uranium Atomic Bomb.
By Fastfission in the public domain.



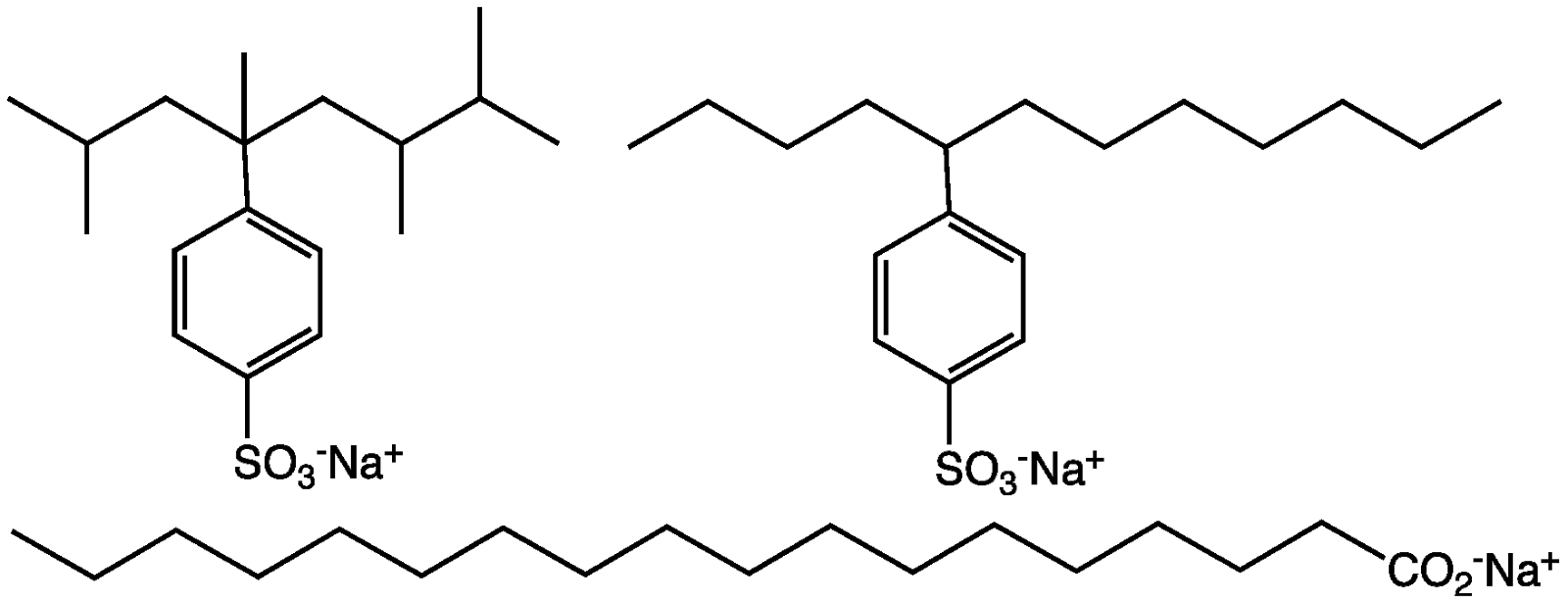


%2B2%2C8.png)



No comments:
Post a Comment